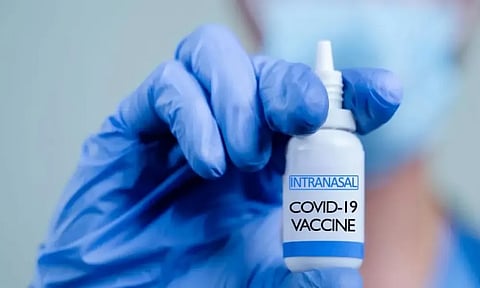
Knocksense Shorts | Bharat Biotech's COVID-19 nasal vaccine gets a nod for restricted use in India
India's first nasal vaccine to combat the spread of COVID-19 infections has been approved for restricted usage by the Ministry of Health’s Central Drugs Standard Control Organisation (CDSCO). Developed by the Bharat Biotech, this recombinant nasal vaccine will be up for use by individuals aged 18 years and above, in emergency situations.
Notably, nasal vaccination is considered effective as it cuts down the cost of needles and syringes and doesn't compulsorily require dependence on trained individuals to supervise the vaccination procedure.
Reportedly, Bharat Biotech successfully completed the clinical trials of the nasal vaccine with about 4,000 volunteers, with zero cases of adverse reactions or side effects.
To get all the latest content, download our mobile application. Available for both iOS & Android devices.